Thanks to all who responded to our survey. There was overwhelming support for a Q4 BioBash.
SAVE THE DATE for Tuesday, November 16, 6-8pm at the Bullock Texas State History Museum*.
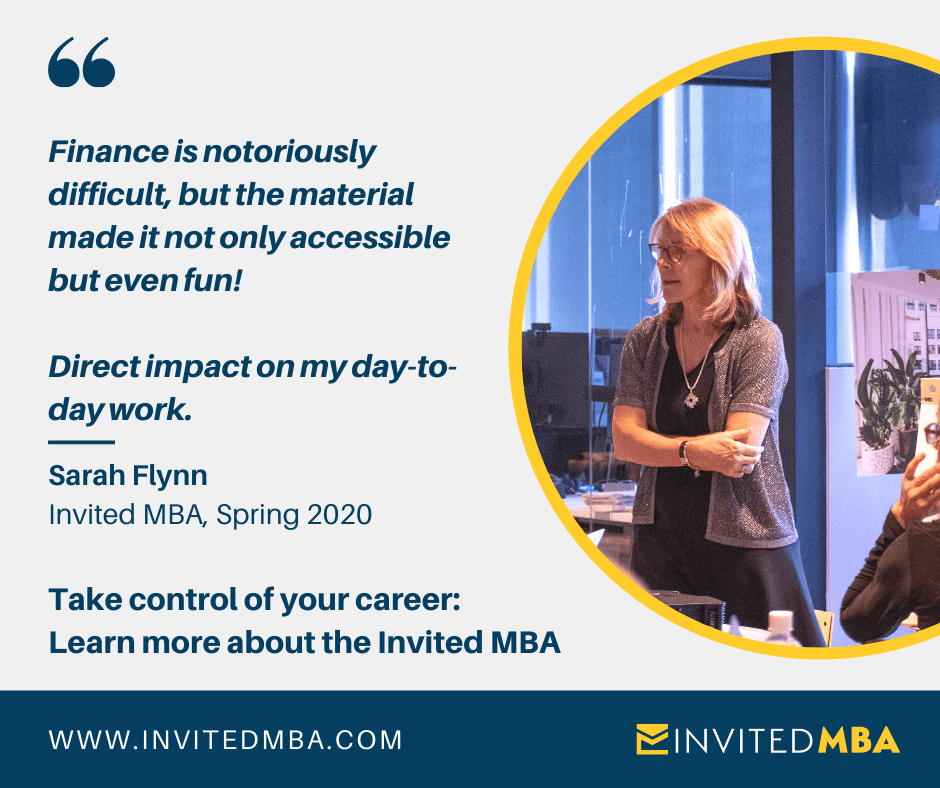
AND a big thanks to our first BioBash sponsor, Austin’s fully virtual Invited MBA. See the Spotlight section for more information. Invited MBA is offering discounts to the BioAustinCTX membership and newsletter readers.
*If Covid restrictions prevent meeting in person, the BioAustinCTX BioBash will be postponed to Q1 2022.
What can we do while we wait? There were several suggestions for smaller in-person or virtual events which are great ideas. In order to bring what you want and need, please answer these few questions.


TMDA Events
REGISTER TODAY
TMDA MedTech Supplier Summit: Market Place Solutions from Bench to Bedside
October 21 from 2pm – 5pm CDT
TMDA CEO Fireside Chat
February 17, 2022 from 2pm – 4pm CDT
Be a Part of the Newsletter
Send us your job postings, news, resources and events to share with the community.
Submit your information today to be a part of next month’s newsletter.

SPOTLIGHT
The Invited MBA is a 12-week, fully virtual mini-MBA program built around award-winning competitive business simulations and action learning projects. We’re offering members of the BioAustin Community (through this newsletter) an exclusive $200 tuition credit. Additionally, the first 10 BioAustinCTX members who are accepted and enroll will be awarded an automatic $1,000 scholarship for the fall cohort, final application deadline is September 9th! Both individual professional members and employees of corporate members qualify.


UPCOMING EVENTS
Local Events
September 9 – HR Round Table: Changes to the Texas Sexual Harassment Law – Hosted by ATC
September 16 – HR Roundtable: The IT Workforce – Overcoming Short and Long Term Challenges – Hosted by ATC
Sept 31 – Oct 1 – BioNTX iC3 Summit 2021 – Reignite! – Hosted by BioNTX
October 1 – Annual BioHouston Chili Cook-off – Hosted by BioHouston
Nov 10 – 11 – 2021 Texas Life Science Forum – Hosted by BioHouston
Partnering with Payers & Health Systems
Exploring different models that payers, providers, and manufacturers employ towards working with small companies; what are the health systems looking for. Free webinar.Aseptic Fill / Finish: A Holistic Approach
This webinar will give you an overview of the challenges and of Fill/Finish including sterilization and how to overcome them by working with SME’s from different companies and achieving a fast and reliable solution for bringing your product fast to the market.Sales & Market Access
Selling into hospitals, when to / how build a sales force or partner with a distributor, best practices for marketing post-COVID. Free webinar.Do You Need a Metallurgist?
The realm of metallurgy has been a part of our existence since humans started extracting metal from the Earth. This presentation will be a series of case studies that illustrate the science and engineering of metallurgy as they apply to real life scenarios.Fundamental Principles for Today’s Clinical Trials
This session is designed to equip senior leadership in clinical operations with a framework to select and evaluate innovation and manage change to optimize clinical trials not only for today but with lasting effect for the future. Rather than reviewing tactical decisions about point solutions, this session will give you tips and insights from key trial stakeholders on how to ensure your evaluation of technology starts with the end in mind and supports faster, safer, more efficient clinical trials.6 steps to overcome labelling challenges in the life sciences industry: How to drive efficiency & control through digital maturity
In this webinar, Kallik’s CEO, Gurdip Singh, and Life Sciences labelling Specialist, Bob Tilling, will guide attendees through their digital maturity journey and how to best use technology to have the right information, on the right label, at the right time. By following the Digital Maturity Curve – a best practice, step-by-step methodology- life sciences manufacturers can take gradual steps into assessing their business landscape and transitioning to digital solutions.Why a Global Labeling System is becoming critical for Medical Device Manufacturers
Today’s global medical device market is creating challenging conditions for device manufacturers. In this information packed session, PRISYM ID speakers will share insight into why continuing with international medical device manufacturing without the support of a true GLS, will create continual challenges, and why now is the time to take a different approach.Device Security
MedTech Innovator is the industry’s nonprofit global competition and accelerator for medical device, digital health and diagnostic companies. Our mission is to improve the lives of patients by accelerating the growth of companies that are transforming the healthcare system. Join them for a free webinar about device security.Understanding the New IEC 60601-1 Amendment 2 Requirements
Medical device standards are updated often. This can make it difficult for medical device manufacturers to keep up with the latest changes, impacting their quality systems and technical documentation, product development schedules, and time to market. Are you ready to meet the new IEC 60601-1 Amendment 2 requirements?Get to Market Summit Series
No matter where you’re at in the process, there’s a lot to plan to successfully get your device in the hands of end users and to maintain regulatory compliance across global markets. From napkin drawing to product launch, industry experts at this event will help guide you through processes, considerations, and regulations to make sure you’re equipped with the knowledge and tips to bring your device to market (and stay there). And it’s free!Increasing Diversity in Clinical Trials
There are many patient communities across various races and ethnicities that are often underrepresented in clinical trials, which creates significant gaps in the safety and efficacy data of new therapies. We’d like to advance the discussion and understand how stakeholders in clinical trials can effectively promote diversity and align with the latest FDA guidance to proactively increase the participation of underrepresented patient populations.Improving inhaler technique with electronics: connected inhalers
During the webinar, participants will gain an understanding of how connected inhalers can help to pave the way to be a win-win situation for patients, the pharmaceutical industry and healthcare providers. Participants will also see how important flow inhalation measurement technology is in response to the most common mistakes while using an inhaler.Accelerating technology transformation to build resilient healthcare enterprises
Join this session with Anant Adya, SVP and Business Head for Cloud, Infrastructure & Security Services, Infosys and other speakers to hear their perspectives on how well-orchestrated technology investments can help build resilient and agile healthcare enterprises.Face the future of manufacturing through predictive maintenance data management towards a more sustainable future with metered dose inhalers
Industrial automation components are subject to wear, that can lead to lower efficiency and performance, increase of energy use or even the complete failure. Predictive maintenance technologies can determine when maintenance should be performed and can provide cost savings over traditional time-based preventive maintenance, because tasks are planned only when necessary.
Towards a more sustainable future with metered dose inhalers
In this webinar, we will explore how the Pharmaceutical industry’s use of propellants has evolved in the drive for greater sustainability including a reduction in the carbon footprint of inhalers, providing some context on the impact pMDIs have on the environment today while at the same time suggesting some areas for improvement.BioNTX iC3 Summit 2021 – Reignite!
Global sourcing for optimised cost and supply in clinical trials
The comparator sourcing industry has doubled over the past five years, with the need for secure and transparent sourcing of comparator and non-investigational medicinal products (NIMPs) expected to continue to rise, globally. Join our webinar to discuss actionable steps for your sourcing strategy to ensure your trial remains in budget and progresses according to expected timelines.
Collaboration Pushing Medical Device Technology Boundaries
Case studies for optimizing design and manufacturing processes in surgical and cardiac devices. Register today to take part of this informative webinar that will increase your toolbox of experience for leveraging existing solutions. Our goal is to make your medical devices consistently more robust while reducing size and maintaining aggressive cost targets.
Innovative Assays for Immunotherapy Drug Discovery Programs
In this webinar, we discuss the key players targeted in immunotherapy and how these cells and processes can be assessed in vitro. We also provide approaches for selecting the most appropriate assay for your target of interest and requirements. In addition to showcasing innovative in vitro assays for immunotherapy drug discovery, this webinar will also summarise the current challenges, considerations, and emerging areas within the field. There will also be a live Q&A session where you’ll be able to put your questions to our expert.
FDA Innovating Patient Access & Payer Task Force
Regulatory strategy at a high-level, FDA initiatives for patient access and payer task force, as well as new trends in the space that can impact early stage companies.
Corporate Social Responsibility and DEI
TMDA Supplier Summit
CEO Summit 2021
TMDA CEO Fireside Chat
Austin Technology Council Calendar of Events
https://tinyurl.com/y9zzr6jvTexas States Small Business Development Center Network Calendar of Events
https://tinyurl.com/ybf68aomWomen in Business Virtual Calendar of Events
View the full list of virtual events.Temple Health & BioScience District’s (THBD’s) E-Learning Series Webinars Library
https://www.templebioscience.org/elearning/NAMSA’s Podcasts Calendar of Events
https://namsa.com/resources/podcasts/biocompchatibility-podcast/
LIFE SCIENCE INDUSTRY NEWS
Local Life Science Headlines
Houston’s robust life sciences ecosystem is about to welcome a new coworking space for early-stage biotech startups. K2bio will offer experienced biotech research managers, staff and other resources in a state-of-the-art facility just south of the 610 loop, including a mix of shared and private research laboratory spaces. The facility officially opens in September.
National News
Effective September 5, Lilly Bio-Medicines will split into two units: Lilly Neuroscience and Lilly Immunology. Anne White, senior VP and president of Lilly Oncology, will lead Lilly Neuroscience as senior VP and president.
Medpace’s newest infectious disease physician discusses how the pandemic might impact future clinical research.
The Food and Drug Administration won’t approve FibroGen’s anemia pill without the company conducting further clinical study on its safety, casting doubt on the medicine’s future in the U.S. The future of roxadustat in the U.S. hinges on what exactly the FDA is requiring, and whether Fibrogen and AstraZeneca are willing to follow through with additional study.
International Industry Interests

INDUSTRY RESOURCES & FUNDING OPPORTUNITIES
The biggest challenge medical device companies face today is bringing quality products to market quickly. Product development teams struggle with losing velocity because of fragmented tools and data sources they must depend on to ensure safe products. We’re excited to announce Design Controls, a new product in Qualio built on ISO 13485 and CFR 820 in harmony with ISO 14971, that enables product development and quality teams to work together and ship high-quality, life-saving products faster.
Are you ready to improve manufacturing output? Are you satisfied with your organization’s current approach to TQM? A bulletproof TQM strategy outlines the strategies manufacturers follow to improve product quality and, by association, customer satisfaction. Without a quality management system (QMS) in place, the process is highly inefficient and laborious, to say the least. Learn more about the major TQM principles medical device startups need to be aware of.
Learn How to Prepare for an FDA Inspection
If FDA called tomorrow to announce their plans to visit your facility next week, would you be prepared? Would you know what you need to do to get ready? Here are 5 tips & pointers to help your company get prepared for an FDA inspection.The Ultimate Guide to CAPA for Medical Devices
The corrective and preventive action (CAPA) process is the heart of any quality management system. And yet, year after year, CAPA is the #1 reason device makers receive 483’s from FDA. This doesn’t have to be the case. Use this guide to help you implement and maintain a healthy CAPA system, in order to avoid the common pitfalls such as being reactive versus proactive, poor root cause determination, and more.3 Life[cycle] Hacks for Integrating Risk Management throughout all Device Phases
Too many companies still treat risk management as a “checkbox” activity. They look up the regulations, only to apply them as a high-level list of to-dos for the team to check off as they develop the product. But this compliance-only mentality doesn’t do risk management justice, and it isn’t the best way to focus on bringing a safe, high-quality medical device to market. Check out this blog to learn how to integrate risk management into everything.Call for Presenters
The Health Cell, San Antonio’s premier networking and leadership development organization for healthcare and bioscience professionals, is calling for presenter nominations for its annual innovative storytelling event. The State of the Industry is a once-a-year opportunity to bring your pioneering work, research, product, or company into the spotlight for a broad audience of regional and global leaders. There are 2 upcoming dates: Tuesday, October 5, 2021 and Thursday, March 24, 2022.Texas Health Catalyst Application
 Attn: Austin area startups & entrepreneurs. Apply to Texas Health Catalyst @ Dell Medical School to receive consulting and customized guidance for your health product idea or discovery. The program is a great starting point for startups looking to explore collaborations with Dell Med clinicians and local healthcare industry mentors. All Austin area companies and entrepreneurs are eligible to apply including UT Austin spinouts. Areas of interest: Therapeutics, Devices, Diagnostics/Research Tools, Digital Health/Software.
Attn: Austin area startups & entrepreneurs. Apply to Texas Health Catalyst @ Dell Medical School to receive consulting and customized guidance for your health product idea or discovery. The program is a great starting point for startups looking to explore collaborations with Dell Med clinicians and local healthcare industry mentors. All Austin area companies and entrepreneurs are eligible to apply including UT Austin spinouts. Areas of interest: Therapeutics, Devices, Diagnostics/Research Tools, Digital Health/Software.

WOMEN IN LIFE SCIENCE & WOMEN’S HEALTH
AWIS Virtual Career Fair
The Association for Women in Science Virtual Career Fair aims to connect AWIS members and all women in science with employers seeking top talent. This aligns with AWIS’ work toward equal inclusion and advancement of women in science positions at all levels, from early career to senior leadership.2021 International Life Science Women’s Conference
The Life Science Women’s Conference (LSWC) creates an environment where women working or desiring to work in all areas of life science companies and academia can gather to network and collaborate with other women with regard to entrepreneurship, professional development, inspiration, funding, career enhancement, scientific and technical updates.The Visionary List: Meet The Women Over 50 Shaping The Future Of Science, Technology And Art
Forbes’ 50 Over 50: Vision list “highlights women over the age of 50 who are bringing breakthrough technologies and creative thinking to science, art, technology and healthcare,” writes Maggie McGrath. The list includes Cedilla Therapeutics President and CEO Alexandra Glucksmann, Pfizer Head of Vaccine R&D Kathrin Jansen, and Harvard University professor of biologically inspired engineering Jennifer Lewis, among other movers and shakers in life sciences, health care, art and other fields.How Humacyte CEO built a successful women-led company
Laura Niklason co-founded regenerative medicine company Humacyte in 2004 with Juliana Blum and Shannon Dahl, but she continued working as a professor of anesthesia and biomedical engineering Yale University until last November, when she stepped up as CEO. Obtaining funds for a company with an all-female executive team in a field that was enduring growing pains at the time wasn’t easy, but the company’s board was built primarily from Niklason’s existing network, and she says she leaned heavily on them.Reducing the gender bias in clinical trials
A study of women’s participation in cardiovascular clinical trials revealed that only 38% of participants were women. But if women comprise 50 percent of the population, that should be reflected in clinical trials too. See how Dr. Ki Park is working hard to make things better for her female patients by dedicating much of her research to women’s cardiovascular health.

Bioscience and Biotechnology Career Fair
The University of Texas at Austin is hosting a virtual career fair for interested employers and graduate programs to meet more than 300 students with experience and interest in the biomedical, biotechnology and life sciences industries. The even is free but you must register by mid-September.Part-time Contract with Mainstream Medical Devices
We are seeking a part-time contractor for Design History File Writing for Class 1 devices. If interested in more information, please contact Dana Wilcox, CEO Mainstream Medical Devices, LLC.Veravas Careers
Do you have the skills and the passion to move diagnostics and precision medicine forward? Learn more about career opportunities at Veravas. We have multiple positions open in the Austin area. Controller Senior Account Executive – Laboratory Segment Senior Account Executive – Pharma Segment Senior Account Executive – Research SegmentCareer Connections
We equip underserved jobseekers with 21st-century skills and connect them to Digital Marketing careers across the US. With a focus on underserved communities, we amplify the online presence of small businesses, while launching the careers of jobseekers.The Global Medical Device Job Board
What if, there was a one-stop shop for candidates and companies looking for top medical device opportunities and talent, worldwide Increase your network with more than 150,000 medical device industry professionals to access exclusive top talent and list incredible jobs in the medical device industry. The job board will always be free to job seekers, and hiring companies will receive their first 90 days of unlimited job postings free.Abbott
Multiple Positions View positions
Aeglea Biotherapeutics
Multiple positions View positions
ALKU
Multiple positions View positions
AMDM IVD Job Board
https://www.amdm.org/jobs.html
Asuragen
Multiple Positions View positions
Austin Technology Council (ATC) Jobs
https://www.austintechnologycouncil.org/jobs/
Babylon Health
Multiple Positions View positions
Black Diamond Networks
Multiple Positions www.blackdiamondnet.com
Commissioning Agents (CAI)
Multiple Positions https://cagents.com/careers
FDA Quality and Regulatory Consultants (FDAQRC)
Luminex
Multiple Positions View positions
Molecular Templates
Multiple positions View positions
Nuclein
Multiple Positions www.nuclein.com/careers/
Skills Alliance
Multiple positions View positions
Syneos Health
Multiple positions View positions

VOLUNTEER OPPORTUNITIES
Austin Chamber Talent Ambassador program
We partner with nearby businesses who offer learning opportunities (guest speakers, internships, apprenticeships, etc.), and connect them to local schools, post-secondary institutions, and those facing barriers to employment.
BioAustinCTX
We are seeking volunteers to help collect and compile data to map the healthcare landscape in Austin. If interested, please fill our volunteer form.

TEXAS LIFE SCIENCE ORGANIZATIONS
ATX Women in MedTech
womeninmedtech@gmail.comAustin Technology Council
https://www.austintechnologycouncil.orgAustin Health Tech Meetup
https://www.meetup.com/Austin-Health-Tech/Austin Technology Incubator (ATI)
https://ati.utexas.eduBeam Founders
https://www.beamfounders.org/BioAustin LinkedIn Group
https://www.linkedin.com/groups/6535301/profileBioMed San Antonio
http://www.biomedsa.orgBioNorth
https://bionorthtx.org/Capital City Innovation (CCI)
https://www.capitalcityinnovation.org/Central Texas Angel Network
https://www.ctan.comHealth Technology Forum Meetup
https://www.meetup.com/HealthTechnologyForu m-Austin/Bio El Paso-Juarez
https://bioelpasojuarez.org/Health Wildcatters, Dallas
www.healthwildcatters.comAustin Chamber of Commerce
https://www.austinchamber.com/economic-development/key-industries/life-science/BioHouston
http://biohouston.org/Temple Health & Bioscience District (THBD)
http://www.templebioscience.com/Texas Healthcare and Bioscience Institute (THBI)
https://www.thbi.com/Texas Biomedical Research Institute, San Antonio
https://www.txbiomed.org/Texas Health Catalyst
https://dellmed.utexas.edu/thcTexas Medical Device Alliance (TMDA)
https://texmda.org/Texas Medical Device Alliance (TMDA) LinkedIn Group
https://www.linkedin.com/groups/6952028The Health Cell, San Antonio
http://thehealthcell.orgTMCX/TMCX+, Houston
http://www.tmc.edu/innovation/innovation- programs/tmcx-plus/Top Austin-Area Medical Device Companies
https://tinyurl.com/ya9v2s5uVelocity Texas, San Antonio
https://velocitytx.org/Women in Bio-Texas (WIB-TX, Austin & Houston)
https://www.womeninbio.org/THANKS TO OUR TMDA SPONSORS

