

How Your Life Science Organization Can Survive and Thrive the Chaos and Complexity of Cybersecurity
November 4, 2021 12pm-1pm CST
BioAustinCTX Lunch and Learn has gone virtual!
Fact: 60% of small businesses fail within 6 months of a breach.
Life sciences organizations, from small to large, can affordably become cyber resilient but many can’t find the right expertise or know where to start.
BioAustinCTX is excited to have Robert Felps, CEO of CyberCompass who has helped life science organizations to get cyber resilient. Robert will share his experience and lead an interactive discussion on cybersecurity challenges and opportunities you are facing. Attendees will have the opportunity to get a quick check on their cyber resilience to understand the exposure your organization is facing.
This virtual lunch and learn event is sponsored by BioAustinCTX and is open to both members and non-members across the great State of Texas. Registration seats are limited so register today.
TMDA Events
REGISTER TODAY
TMDA MedTech Supplier Summit: Market Place Solutions from Bench to Bedside
February 17, 2022 from 2pm – 5pm CDT
Be a Part of the Newsletter
Send us your job postings, news, resources and events to share with the community.
Submit your information today to be a part of next month’s newsletter.

SPOTLIGHT

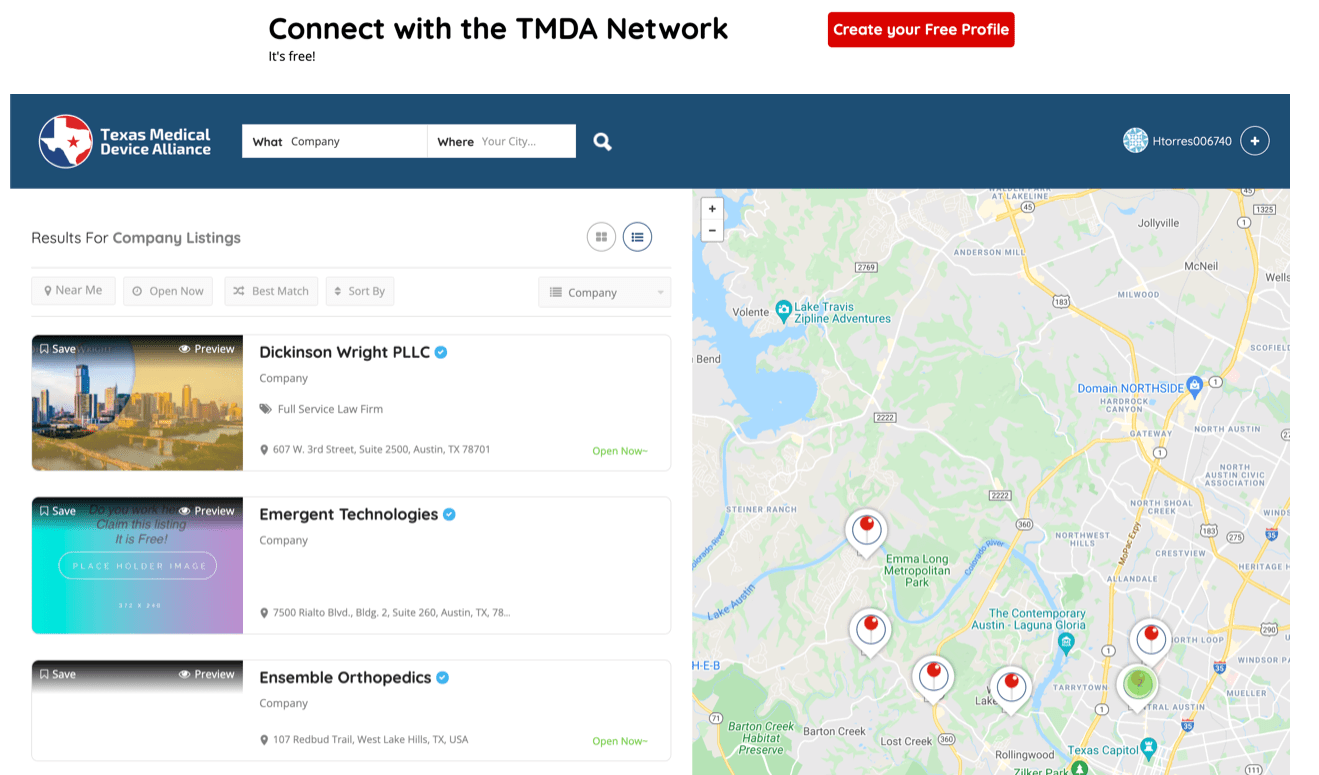
TMDA is thrilled to launch its new Marketplace Network Directory!!
The Texas Medical Device Alliance (TMDA) Board developed an exciting new tool that connects medical device and life science professionals and allows them a space to showcase their companies and services. Called the TMDA Network Directory, participants can enter information on their organizations, including location, contact information, descriptions, videos, demos and more.
This tool is a unique channel to maximize connections, build exposure for your brand, raise awareness and learn about other partners, collaborators and opportunities in the industry. For more information on this free tool, visit www.texmda.org.
How Do I Get Started? It’s easy!
- Just go to texmda.org.
- Click on Create your Free Profile
- Select between a Basic (free), Silver Sponsor ($500), Gold Sponsor ($1000) or Premium Sponsor ($2500)
- Enter information on your business, including map location, address, business tagline, descriptions, images, website, social media information and much more. Business content entry increases with service level purchased. For more information on pricing, please click on https://texmda.org/pricing-plan/
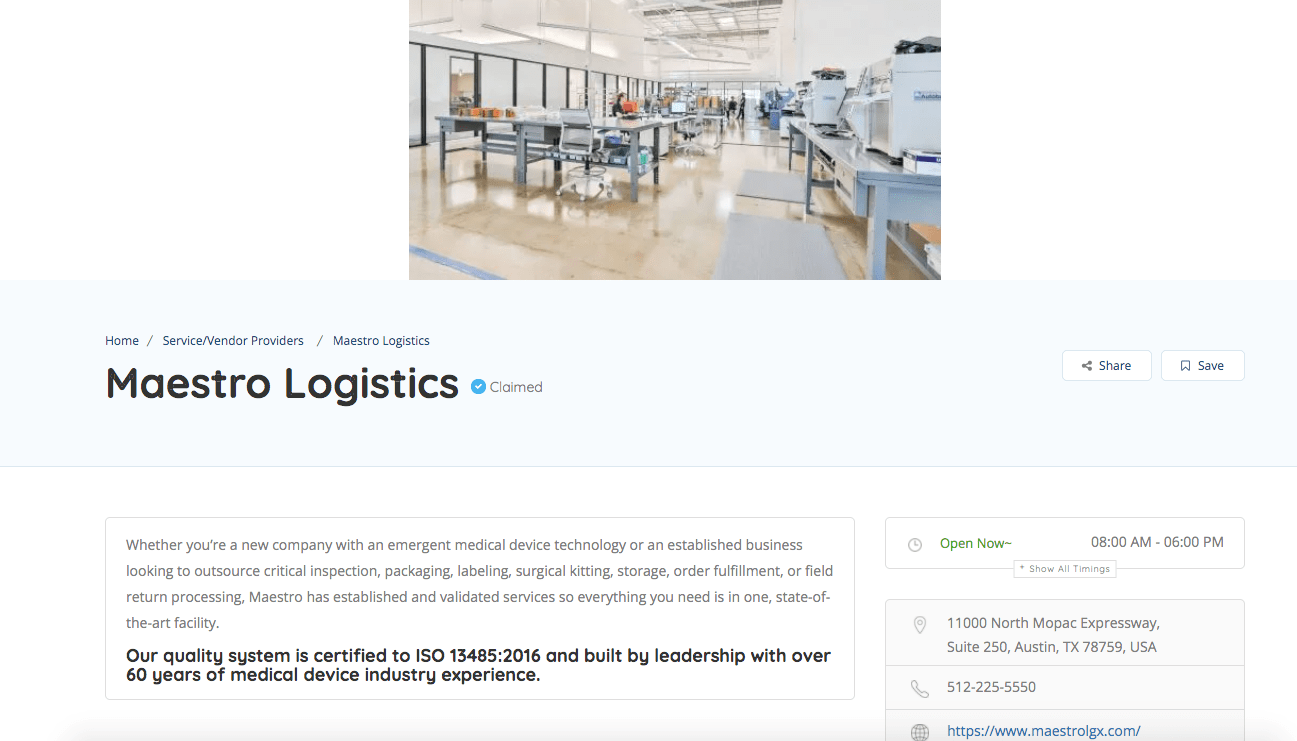
TMDA Directory Sponsor Example
Increase your business awareness and get connected today by creating your profile!
Participants can join as a Company, Individual or Service/Vendor Providers. Their designation will allow for easier searchability. Here is a sample of a Service Provider profile. For more information, please contact us at www.texmda.org.

UPCOMING EVENTS
Local Events
November 4 – How Life Science Organizations Survive the Chaos and Complexity of Cybersecurity – Hosted by BioAustinCTX
November 8 – CEO Summit 2021 – Hosted by ATC
November 9 – Annual Policy Summit – Hosted by THBI
November 10 – 11 – 2021 Texas Life Science Forum – Hosted by BioHouston
November 17 – Disrupt Diabetes Summit – Hosted by Health Tech Austin
The Pulse Breakfast – Accessing Alternative Sources of Capital (IN PERSON)
Bob Rauker, CEO of Plano-based medical device company Belluscura, one of two Dallas-area life science companies to go public in London this summer, will outline the reasoning for and benefits of accessing capital this way in a fireside chat with Rachel Carroll, President & Managing Partner of Edison Group, a global independent equity research and investor relations company.
How to Get the Best from Supplier Audits
Any one vendor can introduce risk to the entire study, its patients, or results. Today’s sponsor must have a philosophy as well as clear procedures to develop and implement a risk-based approach to vendor GCP qualification and auditing. Join us for a roundtable discussion where a panel of experienced auditors will share their expert advice and innovative solutions that avoid problems and help both clinical trial Sponsors and Vendors to manage and prepare for vendor qualification assessments.
Creating Analytics for Business Improvement
Given the ongoing complexity in the healthcare marketplace and the need for constant business performance improvement to reduce costs and increase revenue, new, real time, dynamic tools, and metrics help make decision making easier for those who work in the healthcare industry. We’ll be presenting success stories and thoughtful insights on the ongoing changes in the industry, how the better use of data can improve patient care, drive performance improvement, and increase revenue.
Getting it right! Considerations for immuno-oncology and vaccine studies and the bioanalytical tools used
Choosing the right bioanalytical platform for your immunology or vaccines clinical trial is critical to data collection. But navigating the intricate bioanalytical process and selection of assay platforms can be difficult. What do these platforms look like in use, and how should you go about selecting the right one? This webinar will demystify the bioanalytical requirements for clinical studies and will discuss the most common bioanalytical platforms used during clinical trials to measure vaccine responses and levels of inflammatory mediators in biological fluids.
Biosecurity – What Should We Be Concerned About?
Biosecurity risks are a wide and diverse field that isn’t limited to what’s in your labs. There are many different types of players across the globe. Join our two experts to learn much more.
Panelists: Yong-Bee Lim, PhD, Fellow, Council of Strategic Risks
> Kathleen Vogel, PhD, Director + Professor, School for the Future of Innovation in Society, ASU
Global Entrepreneurship Week Fort Worth
Each November, Global Entrepreneurship Week (GEW) reaches millions of people of all ages and backgrounds through local, national and global events and activities. During the week each November, speakers, panels, competitions, showcases and networking make up the thousands of events held around the world.
CEO Summit 2021
CEO Summit, presented by Workhorse Marketing is an afternoon to evening forum where over 150 of Austin’s leading technology CEOs network, learn about cutting-edge best practices, share expertise with peers, and begin projects that will continue to make Austin a place for small to enterprise tech companies.
ATC is committed to promoting and supporting the growth of the Austin tech ecosystem. In this spirit, the day is filled with panels, breakouts, happy hours, and networking. The CEO Summit is an exclusive event for C-Suite executives only.
Accelerating Clinical Trials with Best-in-Class Technology
Traditional methods of setting up trials and managing large volumes of clinical data rely on time-consuming, repetitive manual processes. Today, our industry is increasingly adopting technology solutions to automate end-to-end clinical trials, from protocol to submission. As a result, it’s vital for organizations to be armed with the right knowledge and strategy when selecting solutions and vendors, in order to avoid pitfalls and pave the way for successful implementation. Register for this webinar to hear thought leaders from Formedix and eClinical Solutions discuss metadata management, study automation, and ultimately how to gain faster insights with Clinical Metadata and Data Repositories.
Annual Policy Summit
Each year, THBI hosts the annual Summit and Luminary Awards events to highlight the world-class innovation taking place across the state and to educate state policy leaders. This fall, we are excited to plan for the first in-person event since the 2019 Fall Summit. This event will include a half day conference and will host conversations highlighting health policy initiatives and issues impacting patient access, research and development, and the future of the healthcare and life sciences industries in Texas. The event will also highlight industry leaders who have furthered innovation in Texas with the presentation of the annual Luminary Awards at a dinner following the Summit. The Luminary Award honors individuals for their contributions and efforts to further the development of the life science industry in Texas.
Move Faster and Break Nothing with Med Devices, SAMD and DTX
This event will feature a mix of industry leaders and pioneers sharing first-hand lessons learned from their pioneering case studies accelerating the development of medical device software at the dawn of the age of digital health. During this webinar, they will share best practices and worst practices learned through their leading edge efforts. They will discuss a variety of tech industry approaches to leverage such as fast feedback loops (e.g., agile and Lean Startup), design thinking, and non-traditional leadership approaches, and how to best fuse those approaches with the constraints and decades of experience already known in regulated MedTech.
Manufactured in the USA: Choosing a US-based CDMO
In this webinar, we’d like to introduce Avéma Pharma Solutions, a contract manufacturer with the capabilities that pharma companies need when selecting a CDMO including in-house R&D capabilities, formulation development, full analytical labs, process scale up and transfer expertise, development and validation of analytical methods, pilot scale and commercial manufacturing capabilities, and regulatory support. As an organization focused on speed to market, we can help you launch your products quickly, efficiently and cost effectively.
Founders Guide to Startup Accounting
Founders Circle is a discussion forum for life science startup founders and C-level executives. This series is facilitated by industry experts and investment professionals and centers on business challenges that all early-stage life science companies face. Discuss innovative growth strategies and learn from the experiences of an exclusive network of your peers.
Disrupt Diabetes Summit
Thought Leaders and Innovators Are Coming Together To Share Their Knowledge, Experience, Research, and Insights about Disrupting Diabetes.
Raising Capital True Quality Summit Series
The Raising Capital True Quality Summit Series is designed to help early-stage medical device, MedTech, and digital health company leaders learn best practices and expectations for raising their first rounds of funding. Discussions will dive into what investors are looking for when they make their decisions, the changes that COVID has made to the investment landscape, how to avoid pitfalls that many first-time founders make on their path to funding, and much more. The day-long, virtual event will feature sessions and panel discussions from investors, partners, strategics, and company leaders who have been through the process.
TMDA MedTech Supplier Summit: Market Place Solutions from Bench to Bedside
Austin Technology Council Calendar of Events
Texas States Small Business Development Center Network Calendar of Events
Women in Business Virtual Calendar of Events
View the full list of virtual events.
Temple Health & BioScience District’s (THBD’s) E-Learning Series Webinars Library
https://www.templebioscience.org/elearning/
NAMSA’s Podcasts Calendar of Events
https://namsa.com/resources/podcasts/biocompchatibility-podcast/

LIFE SCIENCE INDUSTRY NEWS
Local Life Science Headlines
ReCode Therapeutics Raises Oversubscribed Series B Financing Round of $80 Million
4 Examples of Continuous Improvement in Quality Management in Life Sciences Companies
We all know that quality management isn’t a “set it and forget it system,” but how do you actually make continuous improvement in quality management a reality in your life sciences company? We’ve put together some examples of continuous improvement in quality management that provide the inspiration that you need to take your company to the next level.
National News
Digital Control: Putting Solutions Into the Hands of Patients
Patient engagement solutions are key to clinician-patient interaction, improving compliance, enabling real-time reporting, and reducing visits to the doctor and hospital. Hear from industry thought leaders in this free article.
FDA Clears 5-Minute Test for Early Dementia
The US Food and Drug Administration has given marketing clearance to CognICA, an artificial intelligence–powered integrated cognitive assessment for the early detection of dementia. Developed by Cognetivity Neurosciences Ltd, CognICA is a 5-minute, computerized cognitive assessment that is completed using an iPad. The test offers several advantages over traditional pen-and-paper-based cognitive tests, the company said in a news release.
The Best QMS Software: The Pros and Cons of the 5 Top Options
Choosing a quality management software that fits your life science organization’s unique needs is essential to getting to market faster. Here’s an overview and side-by-side comparison of the top 5 QMS options to ensure you make the best decision for your company.
Keytruda Approved as First-Line Advanced Cervical Cancer Therapy
The FDA has approved Merck’s Keytruda as a first-line therapy for advanced cervical cancer. Keytruda (pembrolizumab) can be used in combination with chemotherapy, with or without bevacizumab, to treat patients with persistent, recurrent or metastatic cervical cancer where the tumor expresses a certain level of PD-L1.
International Industry Interests
Cleanroom Standard EN 17141:2020 and Biocontamination: What Do You Need to Know?
EN 17141:2020 Cleanrooms and associated controlled environments – Biocontamination control, introduced in August of 2020, is the new European standard that replaces EN ISO 14698-1:2003 and EN ISO 14698-2:2003. If you distribute medical devices in Europe, overlooking this new standard may mean risking non-compliance. Whether you’re a new startup or an established manufacturer, complying with EN 17141:2020 will help to proactively identify risks, establish and implement a plan, and trend and monitor microbiological test results.
3 FAQ about CE Marking Medical Device Manufacturers Want to Know
Every medical device needs a CE Marking in order to be sold in the EEA, even devices with the lowest-risk.
Before you begin the process of obtaining that CE Marking, there are a few key questions commonly asked by medical device manufacturers that you should know the answers to, especially if you want to fast-track your medical device to market
IEC 62366 Explained: What You Need To Know About Usability Engineering
Usability engineering (UE)—or human factors engineering (HFE), as it’s also known—is focused on designing a user interface (UI) that allows people to quickly understand how to interact with a product in the most efficient and error-free manner. Usability engineering is also a high priority in the medical device industry. Devices that lack an intuitive design and fail to take into account the user-device interaction can jeopardize patient and user safety.
9 Best ISO 13485:2016 Training Programs in 2021
The caliber of your team’s ISO 13485:2016 training has a significant impact on the success and effectiveness of your quality programs. Our quality experts have curated this list of courses that have helped teams like yours dramatically improve their quality management system processes.
Costa Rica Holder Guide
The Costa Rica Registration Holder (CRH) is the physical or legal entity who manages the sanitary registration of an EMB (Equipment or Biomedical Material) or Medical Device and who acts as the primary point of contact with the Ministry of Health (MoH). In addition, the holder is in charge of submitting any regulatory requirement to the authorities. In other words, the CRH plays a key role as your partner during the life cycle of the sanitary registration in Costa Rica. Similarly, the Product Holder is another relevant party involved in this regulatory process. The Product Holder owns the EMB with a specific commercial name. In most cases the Product Holder is the legal manufacturer.

INDUSTRY RESOURCES & FUNDING OPPORTUNITIES
Ultimate Guide to Quality Assurance and Regulatory Affairs in Medical 3D Printing
This document aims to guide users in the medical device industry through every stage of the product development process, from evaluating manufacturing methods and 3D printing technologies to specific regulatory requirements for commercializing and marketing end-use 3D printed medical devices. Throughout the document are Formlabs and Greenlight Guru resources to support users in each step of the process.
World Pharma Packaging Insight 2021
World Pharma is pleased to present a complimentary digital copy of their latest publication. It provides the latest industry thinking in all areas of pharmaceuticals and is essential reading for those who want to stay ahead of the competition.
Call for Presenters
The Health Cell, San Antonio’s premier networking and leadership development organization for healthcare and bioscience professionals, is calling for presenter nominations for its annual innovative storytelling event.
The State of the Industry is a once-a-year opportunity to bring your pioneering work, research, product, or company into the spotlight for a broad audience of regional and global leaders.
Thursday, March 24, 2022.
Texas Health Catalyst Application
 Attn: Austin area startups & entrepreneurs. Apply to Texas Health Catalyst @ Dell Medical School to receive consulting and customized guidance for your health product idea or discovery. The program is a great starting point for startups looking to explore collaborations with Dell Med clinicians and local healthcare industry mentors. All Austin area companies and entrepreneurs are eligible to apply including UT Austin spinouts. Areas of interest: Therapeutics, Devices, Diagnostics/Research Tools, Digital Health/Software.
Attn: Austin area startups & entrepreneurs. Apply to Texas Health Catalyst @ Dell Medical School to receive consulting and customized guidance for your health product idea or discovery. The program is a great starting point for startups looking to explore collaborations with Dell Med clinicians and local healthcare industry mentors. All Austin area companies and entrepreneurs are eligible to apply including UT Austin spinouts. Areas of interest: Therapeutics, Devices, Diagnostics/Research Tools, Digital Health/Software.

WOMEN IN LIFE SCIENCE & WOMEN’S HEALTH
A conversation with co-founder and CEO of Mothers in Science
How to Speak TECH to NON-TECHS
Getting approvals and funding often depends on non-tech leaders understanding you.
Learn 5 Techniques to use tomorrow!
Reesa Woolf, PhD http://www.LinkedIn.com/in/reesawoolfphd
With CEO hire, this Boston biotech boasts an all-woman C-suite
Dana-Farber Cancer Institute’s Luba Greenwood was named Kojin Therapeutics’ first CEO, joining Chief Scientific Officer Kay Ahn, Vice President of Biochemistry Lynn Abel, and founder and Head of Discovery Biology Vasanthi Viswanathan on the startup’s all-female executive team. The company is growing faster than expected, is hiring chemists, biologists and computational biologists, and is putting the finishing touches on a 15,000-square-foot facility in Boston’s Seaport District.
Trailblazing women who broke into engineering in the 1970s reflect on what’s changed – and what hasn’t
Engineering in the U.S. has long been — and continues to be — a male-dominated profession. Fifty years ago, it looked like that might change. In 1970, the percentage of women majoring in engineering was less than 1%. In 1979, that number was 9%. Today, only 21% of engineering majors are women.

Manager of Regulatory Affairs in Austin, TX area
Alafair Biosciences, Inc Seeking Quality Engineer
Nuclein, LLC Seeking a Director of Systems Engineering
- B.S. or M.S. in Engineering or a Life Science with 5-10 years in Systems or Process Engineering involving software and hardware
implementations for life science products, in vitro diagnostics, or medical devices under design control (21 CFR Part 820, ISO 13485) in a regulated environment. - Understand and generate deliverables related to design controls in support of a regulated industry.
- At least 10 years of experience with IVD medical device products
- Background in nucleic acid-based diagnostics technology and experience with next-generation sequencing are highly desirable. Other system development experience in complex clinical diagnostic development environments is a plus.
- Excellent verbal and written communication skills.
- Enjoy working independently and collaboratively in a fast-paced environment and able to adapt to change.
- Experience in and success with influencing and working through others to achieve results within in a complex matrix organization.
Attogene Corporation Seeking Lab Technician (Contract Positions)
Previous experience in a laboratory environment as a Lab Assistant or Junior Lab Tech
Familiar with following technologies: Lateral Flow, ELISA, Enzyme assays, PCR, Nucleic Acid isolation, Cell culture
Deadline to apply: 11/30/2021
Genotox Laboratories Seeking Lab Technical Supervisor
Lab technical supervisor reports to the Genetic testing lab Manager and is responsible for managing the genetic testing service workflow to ensure the test accuracy and efficiency. Lab technical supervisor provides leadership and support to the genetic testing service team.
The Genetic Testing Lab Technical Supervisor should have proficient knowledge in the following areas:
DNA and RNA extraction
PCR and real time PCR
Biological sample handling
Basic experimental design
Molecular Biology
CLIA requirements
Must have a Bachelor’s degree in life science or post-graduate degree (preferred)
Cassava Sciences Senior Director/Director of Regulatory Affairs
- BS Degree in a scientific discipline is required; an advanced degree (Masters, PharmD, PhD) is desirable.
- A minimum 8-10 years of experience in Regulatory Affairs is required. Experience in development of neurology products is a plus.
- Comprehensive knowledge of applicable regulations and experience with requirements for IND/CTA, NDA/MAA, and interactions with Health Authorities is required.
- Proven ability to work successfully and influence within a cross-functional team/partnership environment with a high level of professionalism.
QA Consulting, Inc. Seeking Sterilization Subject Matter Expert Consultant
cleanroom validation, and cleaning/disinfection validation for reusable medical devices within a Quality Management System
framework. This position requires independent utilization of sound validation techniques and strategies, including but not
limited to ethylene oxide, radiation, and steam sterilization methods, understanding of principles of cleanroom validation and
validation of disinfection and cleaning methods for reusable devices. Must possess a keen understanding of industry
standards and guidance to assist clients with root cause failure investigations in sterilization, and biocompatibility. The
Consultant must interact with other consultants, clients, laboratories, and experts outside QA Consulting to communicate and
implement company and client objectives. Client and project management skills are required. Contact for more information
PharPoint Research Seeking Biostatisticians
– One year related work experience in a clinical research environment
– Knowledge of the drug development process and FDA and ICH guidelines
– Knowledge of basic statistical design, analysis, and programming techniques utilized in clinical research
– Knowledge/experience with Statistical software packages (SAS preferred)
– Remote applicants considered
Career Connections
We equip underserved jobseekers with 21st-century skills and connect them to Digital Marketing careers across the US. With a focus on underserved communities, we amplify the online presence of small businesses, while launching the careers of jobseekers.
The Global Medical Device Job Board
What if, there was a one-stop shop for candidates and companies looking for top medical device opportunities and talent, worldwide Increase your network with more than 150,000 medical device industry professionals to access exclusive top talent and list incredible jobs in the medical device industry. The job board will always be free to job seekers, and hiring companies will receive their first 90 days of unlimited job postings free.
Abbott
Multiple Positions
View positions
Aeglea Biotherapeutics
Multiple positions
View positions
ALKU
Multiple positions
View positions
AMDM IVD Job Board
https://www.amdm.org/jobs.html
Asuragen
Multiple Positions
View positions
Austin Technology Council (ATC) Jobs
https://www.austintechnologycouncil.org/jobs/
Babylon Health
Multiple Positions
View positions
Black Diamond Networks
Multiple Positions
www.blackdiamondnet.com
Commissioning Agents (CAI)
Multiple Positions
https://cagents.com/careers
FDA Quality and Regulatory Consultants (FDAQRC)
Luminex
Multiple Positions
View positions
Molecular Templates
Multiple positions
View positions
Nuclein
Multiple Positions
www.nuclein.com/careers/
Skills Alliance
Multiple positions
View positions
Syneos Health
Multiple positions
View positions

VOLUNTEER OPPORTUNITIES
UT needs your help
Austin Chamber Talent Ambassador program
We partner with nearby businesses who offer learning opportunities (guest speakers, internships, apprenticeships, etc.), and connect them to local schools, post-secondary institutions, and those facing barriers to employment.
BioAustinCTX
We are seeking volunteers to help collect and compile data to map the healthcare landscape in Austin. If interested, please fill our volunteer form.

TEXAS LIFE SCIENCE ORGANIZATIONS
ATX Women in MedTech
Austin Technology Council
https://www.austintechnologycouncil.org
Austin Health Tech Meetup
https://www.meetup.com/Austin-Health-Tech/
Austin Technology Incubator (ATI)
Beam Founders
BioAustin LinkedIn Group
https://www.linkedin.com/groups/6535301/profile
BioMed San Antonio
BioNorth
Capital City Innovation (CCI)
https://www.capitalcityinnovation.org/
Central Texas Angel Network
Health Technology Forum Meetup
https://www.meetup.com/HealthTechnologyForu m-Austin/
Bio El Paso-Juarez
Health Wildcatters, Dallas
Austin Chamber of Commerce
https://www.austinchamber.com/economic-development/key-industries/life-science/
BioHouston
Temple Health & Bioscience District (THBD)
http://www.templebioscience.com/
Texas Healthcare and Bioscience Institute (THBI)
Texas Biomedical Research Institute, San Antonio
Texas Health Catalyst
https://dellmed.utexas.edu/thc
Texas Medical Device Alliance (TMDA)
Texas Medical Device Alliance (TMDA) LinkedIn Group
https://www.linkedin.com/groups/6952028
The Health Cell, San Antonio
TMCX/TMCX+, Houston
http://www.tmc.edu/innovation/innovation- programs/tmcx-plus/
Top Austin-Area Medical Device Companies
Velocity Texas, San Antonio
Women in Bio-Texas (WIB-TX, Austin & Houston)
THANKS TO OUR BIOAUSTINCTX SPONSORS
THANKS TO OUR TMDA SPONSORS

Amy Shepherd, Christine Scheve, Dana Abramovitz, Elisa Maldonado-Holmertz, Gretchen Upton, Joe Skraba, Lance Anderson, Ryan Baird, Sandie Roth, Scott Collins, and Tim Sullivan, on behalf of BioAustin, Texas Medical Device Alliance (TMDA), and Austin Health Technology (AHT)


